Introduction
Maternal and newborn health outcomes in resource-rich countries, particularly the United States, lag significantly behind global standards, with a concerning proportion of pregnancy-related deaths deemed preventable [1]. The consequences of severe maternal morbidity and mortality extend beyond individual health, impacting families and highlighting systemic gaps in healthcare access and delivery [2]. A persistent challenge is ensuring all pregnant individuals receive consistent, evidence-based, and comprehensive maternity care [1]. Furthermore, healthcare providers often navigate complex clinical decisions with insufficient information and limited guidelines, a situation exacerbated by the historical exclusion of pregnant and lactating individuals from crucial clinical trials [3].
Clinical Decision Support Systems (CDSS) have emerged as a promising solution to bridge this gap in pregnancy care. Recognized by organizations like the American College of Obstetricians and Gynecologists, CDSS are increasingly integrated into clinical workflows and Electronic Health Records (EHRs) to guide patient management [4]. Pregnancy care is inherently complex, involving diverse data types (clinical findings, imaging, genetic testing), multiple specialties (obstetrics, gynecology, neonatology), and extended care episodes spanning preconception to postpartum. Effective CDSS in this domain must possess robust EHR interoperability and clinical validity to provide precise and timely support. Beyond improving individual patient care, CDSS are vital for fostering evidence-based medicine and advancing towards a learning health system, connecting the growing wealth of digital health data with actionable knowledge for therapeutics and patient-centered care [5, 6].
The rapid advancement of Artificial Intelligence (AI) and biomedical informatics has ushered in a new era for clinical medicine. In healthcare, AI broadly refers to computer systems designed to mimic cognitive functions associated with human clinical expertise [7]. AI’s integration into CDSS has become increasingly significant, enhancing areas such as knowledge discovery, diagnostic precision, risk prediction, chronic disease management, and patient monitoring [8]. In pregnancy care, the exploration of AI-augmented CDSS holds immense potential to streamline clinical workflows and elevate patient management. However, the sheer volume of clinical guidelines, diverse AI techniques, and varied EHR systems creates a complex landscape. The specific capabilities and characteristics of AI-driven CDSS that can most effectively transform pregnancy care remain largely undefined. While AI applications in obstetrics and gynecology are expanding [9-11], a focused review on AI-augmented CDSS specifically tailored for pregnancy care is crucial.
There is a pressing need for systematic reviews to address critical gaps in the current literature. Decisions in maternal health are often preference-sensitive and based on limited evidence, demanding CDSS designs that incorporate clinical guidelines, carefully curated data, and patient preferences. The dynamic nature of these design elements across different stages of pregnancy necessitates systematic investigation. Moreover, the landscape of AI in CDSS for pregnancy care has evolved significantly in recent years. A systematic review is essential to update our understanding of state-of-the-art AI methodologies in this field and compare them to previous approaches. Existing reviews often concentrate on AI method evaluation and model performance metrics (prediction accuracy, etc.) but overlook the crucial aspect of real-world implementation and external validity. This missing perspective hinders our understanding of the true clinical impact of CDSS.
This article addresses these gaps by presenting a systematic review of empirical studies focused on automated risk assessment tools for pregnancy care and other AI-augmented CDSS. Our aim is to identify challenges and opportunities in leveraging these technologies to improve pregnancy care, using the framework of participants, interventions, comparisons, outcomes, and study design. Specifically, we endeavor to: (1) pinpoint key areas in maternal care where automated risk assessment tools and AI-augmented CDSS are impactful, (2) characterize the current functionalities of these systems, and (3) identify limitations, challenges, and future directions for research and implementation in this vital field.
Methods
This literature review adheres to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [12]. The PRISMA checklist is available in Multimedia Appendix 1.
Bibliographic Database Search
We conducted searches across three major electronic bibliographic databases: PubMed/MEDLINE (including MEDLINE and PubMed Central [PMC]), Embase, and ACM Digital Library. PubMed/MEDLINE is a comprehensive resource for life sciences and biomedical literature, curated by the U.S. National Library of Medicine. PMC acts as a digital archive for biomedical and life sciences full-text journals. Embase is a specialized database focusing on pharmacovigilance. The ACM Digital Library is a comprehensive database covering computing and information technology, including biomedical informatics and digital health applications. Access to Embase and ACM Digital Library was facilitated through the University of South Carolina’s licenses.
Search Strategy Formulation
In PubMed/MEDLINE, we employed “text word” searches, encompassing titles, abstracts, MeSH terms, and keywords, to capture relevant articles. We also utilized [MeSH Major Topic] searches to ensure comprehensive coverage of key concepts. The search strategies were adapted for Embase and ACM Digital Library due to variations in indexing and field terminologies across databases. We limited the search to publications up to and including 2022, with no language restrictions applied. Search strings were designed to encompass pregnancy procedures, pregnancy outcomes, CDSS models, CDSS methods, and AI methodologies (search strings and criteria detailed in Multimedia Appendix 2). Electronic database searches were completed in January 2023. The search strategy was refined by two authors (CL and TL) with input from the broader author team, and the searches were executed by NG.
Study Eligibility and Bias Assessment
Initial deduplication of search results across databases was performed by NG, using PubMed Identifier, titles, publication details, and author information to identify unique publications. Eligibility screening was conducted based on the following inclusion criteria: empirical studies that (1) developed or tested AI methods, (2) developed or tested CDSS or CDSS components, and (3) focused on pregnancy care. Study quality and bias assessment were conducted using criteria adapted from the Risk of Bias 2 tool [13], evaluating (1) the empirical nature of the study, (2) the central focus on “pregnancy care,” “CDSS,” and “AI,” and (3) the completeness, clarity, and validity of reported methods, results, and conclusions. Full-text reviews were conducted for potentially relevant studies to determine final inclusion. Two reviewers (TL and NG) independently assessed candidate publications for inclusion and quality. Discrepancies were resolved through discussion with a senior reviewer (CL), leading to a final set of 30 studies selected for review (study selection process illustrated in Figure 1).
Figure 1. PRISMA flowchart. AI: artificial intelligence; PRISMA: Preferred Reporting Items for Systematic reviews and Meta-Analyses. Note: Under “Reports excluded,” exclusion reasons #1, #2, and #3 are not mutually exclusive.
Data Synthesis and Analysis
Where multiple publications reported ancillary information or overlapping outcomes from a single study, these were treated as a single study unit. Two independent coders (CL and TL) extracted the following data points from each study: authors and year, study objectives, pregnancy care applications, CDSS functionality, data source, study population, AI methods, CDSS performance, validation methods, and implementation details. Pregnancy care applications were categorized into prenatal and early pregnancy care, obstetric care, and postpartum care. Obstetric complications were defined to include maternal (e.g., perinatal hemorrhage, ectopic pregnancy, gestational diabetes), fetal (e.g., miscarriage, stillbirth, preterm birth), and neonatal (e.g., bradycardia) adverse events. These categories are not mutually exclusive. CDSS functionality was categorized, with clinical prediction defined as the prediction of adverse events, outcomes, prognosis, and risk identification. Validation types were classified as internal (validation within the study context using data from a homogeneous dataset) and external (validation using data from different contexts to assess generalizability).
Results
Study Selection and Overview
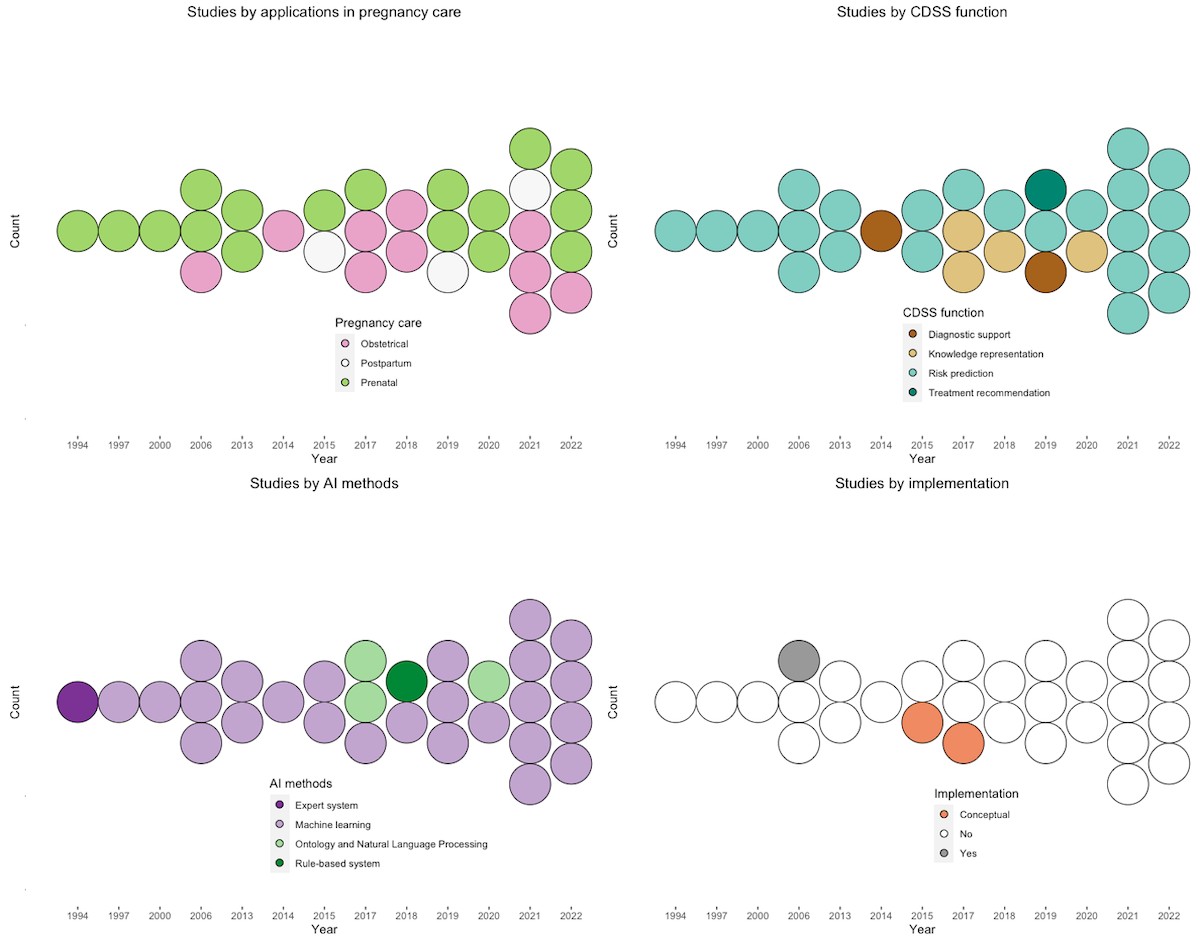
Our search identified 206 studies from PubMed/MEDLINE, 101 from Embase, and 377 from ACM Digital Library. After removing duplicates and applying exclusion criteria, 30 unique studies met the eligibility criteria (Figure 1). Analysis of these 30 studies revealed key themes, summarized in Table 1. The number of relevant publications has generally increased over time, with a temporary decrease between 2013 and 2014 (Figure 2).
Table 1. Summary of reviewed studies.
| Study | Study objectivesa | CDSSb functions | Data source | Sample | AIc methods | Performance | Validation | Implementation |
|---|---|---|---|---|---|---|---|---|
| Woolery and Grzymala-Busse (1994) [14] | Expert system for preterm birth risk assessment. | Risk prediction | Registry (multiple sites, United States) | 18,890 cases | Expert system, machine learning | ACCd 53%-88% | External | No |
| Mongelli et al (1997) [15] | Develop an expert system for the interpretation of fetal scalp acid-base status. | Risk prediction | Scalp blood samples (single, England) | 2174 samples | Logistic transformations, back-propagation networks, decision tree | N/Ae | Internal | No |
| Goodwin et al (2000) [16] | Predict preterm birth. | Risk prediction | EHRf (single, United States) | 19,970 patients | Rule induction, logistic regression, neural network | Customized (AUCg 0.75) | Internal | No |
| Catley et al (2006) [17] | Obstetrical outcome estimations in low-risk maternal populations. | Risk prediction | Registry (37 sites, Canada) | 48,000 cases | ANNh | ROCi 0.73 | Internal | No |
| Mueller et al (2006) [18] | Identify predictors to optimize extubation decisions for premature infants. | Risk prediction | EHR (single, United States) | 183 infants | ANN, multiple layer regression | AUC >0.9 | Internal | Yes |
| Gorthi et al (2009) [19] | Predict pregnancy risk based on patterns from clinical parameters. | Risk prediction | Synthetic cases | 200 cases | Decision tree | ACC 82.5 | Internal | No |
| Ocak (2013) [20] | Assess fetal well-being. | Risk prediction | Cardiotocogram (single, United States) | 1831 samples | SVMj | ACC 99.3% | Internal | No |
| Yılmaz and Kılıkçıer (2013) [21] | Determine the fetal state using cardiotocogram data. | Risk prediction | Cardiotocogram (single, United States) | 2126 samples | LS-SVMk | ACC 91.62% | Internal | No |
| Spilka et al (2014) [22] | Examine cardiotocogram and support decision-making (outcomes: diagnostics and risk). | Diagnostic support | Cardiotocogram (single, United States) | 634 samples | Latent class analysis | N/A | Internal | No |
| Jiménez-Serrano et al (2015) [23] | Detect the postpartum depression during 1st week after childbirth. Toward a mobile health app. | Risk prediction | Registry (7 sites, Spain) | 1880 women | Logistic regression, naïve bayes, SVM, ANN | ANN (ACC 0.79) | Internal | Conceptual |
| Ravindran et al (2015) [24] | Assess fetal well-being. | Risk prediction | Cardiotocogram (single, United States) | 2126 samples | Ensemble: k-NNl, SVM, Bayesian network, and ELMm | ACC 93.61% | External | No |
| Paydar et al (2017) [25] | Predict pregnancy outcomes among systemic lupus erythematosus-affected pregnant women. | Risk prediction | EHR (single, Iran) | 149 pregnant women | MLPn, RBFo | MLP (ACC 0.91) | Internal | Conceptual |
| Dhombres et al (2017) [26] | Develop a knowledge base for ectopic pregnancy. | Knowledge representation | Ultrasound (single, England) | 4260 records | Ontology, NLP | Precision 0.83 | Internal | No |
| Maurice et al (2017) [27] | Develop a new knowledge base intelligent system for ultrasound imaging. | Knowledge representation | PubMed (single, United Kingdom) | N/A | Ontology, NLP | F 0.71 | Internal | No |
| Fergus et al (2018) [28] | Classify cesarean section and vaginal delivery. | Risk prediction | Registry (single, Czechia) | 552 pregnancies | Ensemble: RF, SVM, decision tree, ANN, deferred acceptance | Ensemble (AUC 0.96) | Internal | No |
| Seitinger et al (2018) [29] | Arden Syntax as medical knowledge representation and processing language in obstetrics. | Knowledge representation | N/A | N/A | Arden syntax | N/A | N/A | No |
| De Ramón Fernández et al (2019) [30] | Develop a decision support system to make suggestions for early treatment for ectopic pregnancy. | Treatment recommendation | EHR (single, Spain) | 406 tubal ectopic pregnancies | Multilayer perception, decision rule, SVM, Naïve Bayes | SVM (ACC 0.96) | Internal | No |
| Wang et al (2019) [31] | Develop a postpartum depression prediction model using EHR. | Risk prediction | EHR (single, United States) | 179,980 pregnancies | Logistic regression, SVM, decision tree, Naïve Bayes, XGBp, RFq | SVM (AUC 0.79) | Internal | No |
| Liu et al (2019) [32] | Predict pregnancies. | Diagnostic support | Mobile app | 65,276 women | Logistic regression, LSTMr | AUC 0.67 | External | No |
| Ye et al (2020) [33] | Predict GDMs and compare their performance with that of logistic regressions. | Risk prediction | EHR (single, China) | 22,242 singlet pregnancies | Gradient Boosting Decision Tree, AdaBoost, LightGBM, logistic regression, voting, XGB, decision tree, RF, logistic regressiont | GBDTu (AUC 0.74, 95% CI 0.71-0.76) | Internal | No |
| Silva et al (2020) [34] | Develop readable and minimal syntax for a web CDSS for antenatal care guidelines. | Knowledge representation | N/A | N/A | Ontology | N/A | No | No |
| Venkatesh et al (2021) [35] | Predict the risk of postpartum hemorrhage at labor admission. | Risk prediction | EHR (Consortium on Safe Labor, United States) | 228,438 deliveries | RF, XGB, logistic regressiont, lasso regressiont | XGB (C statistic 0.93; 95% CI 0.92-0.93) | External (multi-site, multi-time) | No |
| Tissot and Pedebos (2021) [36] | Test embedding strategies in performing risk assessment of miscarriage before or during pregnancy. | Risk prediction | EHR (InfoSaude, Brazil) | 4676 pregnancies | Machine learning, ontology embedding | KRALv (F 0.76) | Internal | No |
| Escobar et al (2021) [37] | Predict risk of maternal, fetal, and neonatal events. | Risk prediction | EHR (15 sites, United States) | 303,678 deliveries | Gradient boosted, logistic regressiont | Gradient boosted (AUC 0.786) | External | No |
| Tao et al (2021) [38] | Construct a hybrid birth weight predicting classifier. | Risk prediction | EHR (single, China) | 5759 pregnant women | LSTM, CNNw, RF, SVM, BPNNx, logistic regression | Hybrid LSTM (MREy 5.65 ± 0.4) | Internal | No |
| Mooney et al (2021) [39] | Examine RF to predict the occurrence of hypoxic-ischemic encephalopathy | Risk prediction | Registry (2 sites, Sweden) | 53,000 deliveries | RF | RF (MCCz 0.63) | Internal | No |
| Du et al (2022) [40] | Predict gestational diabetes mellitus. | Risk prediction | Registry (single, Ireland) | 565 women | XBG, AdaBoost, SVM, RF, logistic regression | SVM (AUC 0.79) | Internal | No |
| Schmidt et al (2022) [41] | Predict adverse outcomes in patients with suspected preeclampsia | Risk prediction | Ultrasound (single, Germany) | 1647 patients | Gradient Boosting Decision Tree, RF | GBTree (AUC 0.81) | Internal | No |
| De Ramón Fernández et al (2022) [42] | Predict mode of delivery: cesarean section, eutocia vaginal delivery, instrumental vaginal delivery. | Risk prediction | Registry (single, Spain) | 10,565 records | MLP, RF, SVM | ACC >90 | Internal | No |
| Hershey et al (2022) [43] | Predict spontaneous preterm birth. | Risk prediction | Surveys, biospecimen (10 centers) | 2390 women | SVM | AUC 0.75 | Internal | No |

aThe outcomes of a CDSS model are given in italics.
bCDSS: clinical decision support system.
cAI: artificial intelligence.
dACC: accuracy.
eNot applicable.
fEHR: electronic health record.
gAUC: area under the receiver operating characteristic curve.
hANN: artificial neural network.
iROC: receiver operating characteristic curve.
jSVM: support vector machine.
kLS-SVM: least-squares support vector machine.
lk-NN: k-nearest neighbors.
mELM: extreme learning machine.
nMLP: multilayer perceptron neural network.
oRBF: radial basis functions neural network.
pXGB: XGBoost.
qRF: random forest.
rLSTM: long-short term memory.
sGDM: gestational diabetes.
tBenchmark algorithm
uGBDT: gradient-boosted decision tree.
vKRAL: knowledge representation and artificial learning.
wCNN: convolutional neural network.
xBPNN: back propagation neural network.
yMRE: mean relative error.
zMCC: Matthew’s correlation coefficient.
Figure 2. Trends in reviewed studies. Top-left: trends in studies by applications in pregnancy care. Top-right: trends in studies by CDSS function. Bottom-left: trends in studies by AI methods. Bottom-right: trends in studies by implementation. AI: artificial intelligence; CDSS: clinical decision support system.
Risk of Bias Assessment
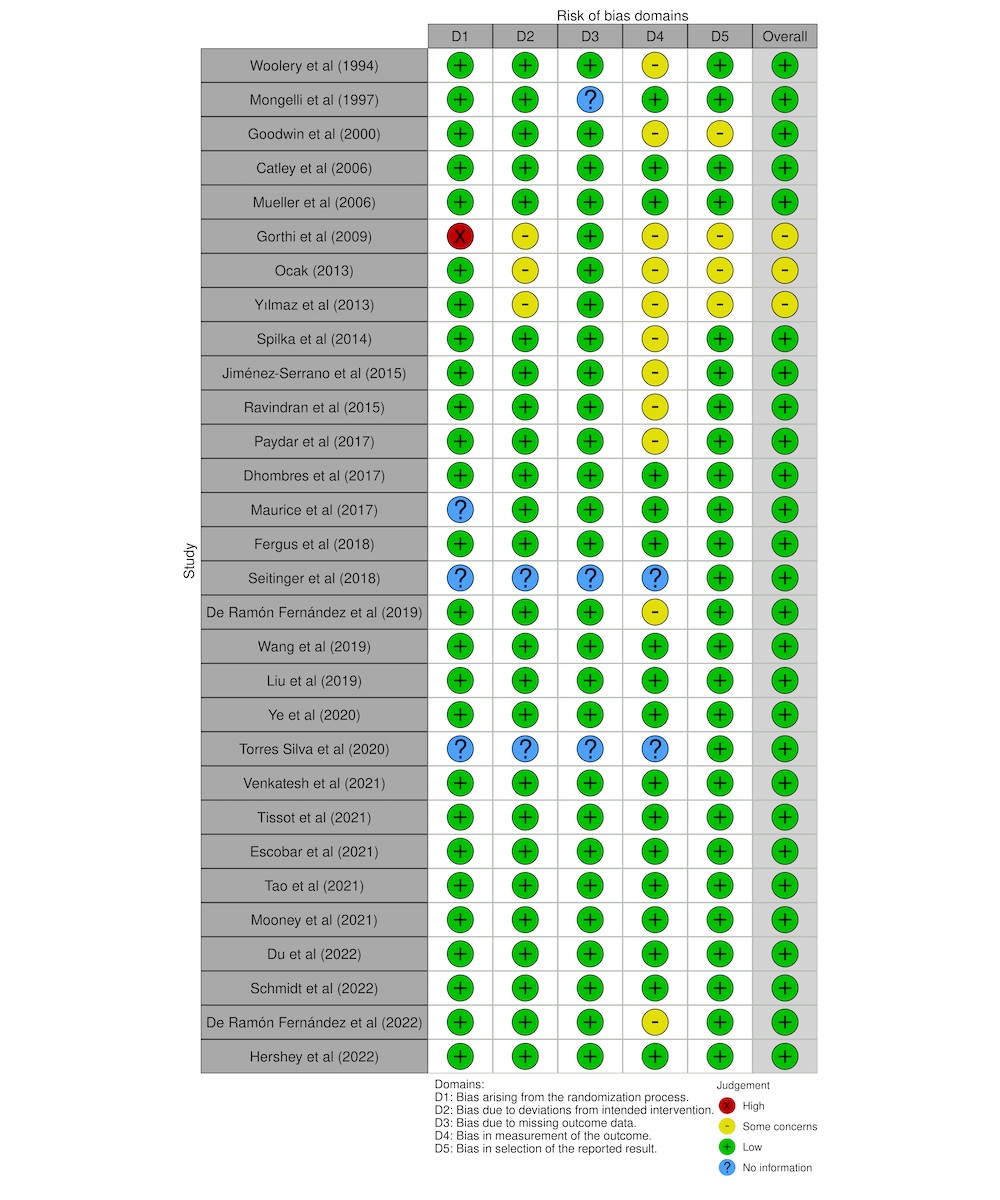
Out of 109 screened studies, 22 were excluded for not being empirically based, 37 for lacking a primary focus on pregnancy care, and 22 for not developing or applying AI methods despite mentioning AI-related terms. This rigorous assessment resulted in 30 studies achieving full agreement on quality between reviewers (TL and NG), following discussions with a third reviewer (CL). Figure 1 details the numbers of studies included and excluded at each stage. Bias risk assessment, following PRISMA guidelines, is summarized in Figure 3.
Figure 3. Traffic light plot for risk-of-bias assessment of included studies.
Applications of Automated Risk Assessment Tools in Pregnancy Care
Prenatal and Early Pregnancy Care: Proactive Risk Management
Automated risk assessment tools are most critically applied during prenatal care for the early detection of maternal and fetal risk factors and abnormalities, enabling timely preventative and interventional strategies (n=17, 57%). CDSS applications in this area include predicting gestational diabetes mellitus [33, 40], miscarriage [25, 36], and adverse outcomes from preeclampsia [41] using data from medical history and prenatal visits. Ectopic pregnancy, a life-threatening condition, is another key area for automated risk assessment. CDSS are being developed to support clinical decision-making after diagnosis, guiding treatment choices to minimize complications [30].
Obstetrical Care: Ensuring Safer Deliveries
With persistently high and rising rates of maternal morbidity and mortality in the US and globally [44], there’s an increasing focus on automated risk assessment tools for obstetric care. Predictive models are being developed to identify individuals at high risk of adverse events, facilitating timely prevention and intervention (n=10, 33%). For example, identifying women at risk of preterm birth allows for advanced care planning during prenatal and perinatal stages [14, 17, 43]. These studies often analyze risk factors using machine learning-based feature ranking to pinpoint data strongly indicative of adverse outcomes. Another significant application is computer-assisted cardiotocography (CTG) interpretation, used during labor and delivery to aid clinical decision-making [21, 22, 28].
Postpartum Care: Addressing Post-Delivery Risks
Automated risk assessment is also crucial in postpartum care, particularly for estimating the risk of postpartum hemorrhage upon labor and delivery admission (n=3, 30%). Postpartum hemorrhage remains a leading cause of maternal morbidity and mortality [45]. Traditional risk assessment relies on stratifying risk factors in medical records using parametric statistical models. However, recent CDSS research is exploring methods to incorporate more nuanced factors beyond established risk factors [35], potentially improving individual risk interpretation and reducing biases inherent in traditional guidelines. Similarly, automated risk assessment tools are being developed for postpartum depression screening, a prevalent yet often underdiagnosed condition [23, 31].
Functionality of Automated Risk Assessment Tools and CDSS in Pregnancy Care
Diagnostic Support: Enhancing Clinical Accuracy
Diagnostic support has been a core function of CDSS since their inception (n=2, 7%) [46]. In pregnancy care, this function is vital for assisting in CTG interpretation [21, 22, 28], as CTG readings are notoriously variable between reviewers. Accurate CTG interpretation is crucial for informed clinical decisions during prenatal care and labor, such as determining the necessity of cesarean versus vaginal delivery. Diagnostic support is also being explored for pregnancy identification using mobile device data [32], offering potential for family planning and preventative care.
Clinical Risk Prediction: Proactive Intervention Strategies
Clinical risk prediction, while less prominent in early CDSS, has become a central function in pregnancy care (n=22, 73%). Automated risk assessment tools are widely used for the early detection of adverse maternal and fetal events. These applications focus on predicting events where early detection of abnormalities allows for timely prevention and intervention, including eclampsia and preeclampsia [37, 41], gestational diabetes [33, 40], preterm birth [14, 17], miscarriage [25, 36], perinatal hemorrhage [35, 37, 41], hypoxic-ischemic encephalopathy [39], low birth weight [38], and postpartum depression [23, 31]. EHRs and medical images are commonly used to train these predictive models, with a smaller number of studies utilizing mobile app data [23, 32].
Therapeutics Recommendation: Guiding Delivery Decisions
Delivery mode selection is a critical clinical decision in obstetric care (n=2, 7%). The increasing rate of cesarean deliveries and their associated risks for both fetal and subsequent maternal outcomes [47] highlight the need for decision support. CDSS are being tested to provide recommendations among cesarean section, eutocic vaginal delivery, and instrumental vaginal delivery using machine learning approaches [42].
Knowledge Base: Foundation for Intelligent Systems
CDSS can be categorized as knowledge-based or knowledge-independent (n=4, 13%). Several studies have focused on developing knowledge bases to underpin pregnancy care CDSS. These knowledge bases utilize formats like Arden syntax and ontologies for representing clinical guidelines and medical knowledge graphs, and XML for web and mobile CDSS applications. Ontologies are used in diagnostic and therapeutic support for ectopic pregnancy, particularly for annotating medical images like obstetric ultrasounds [26, 27]. Arden syntax formalizes obstetric clinical guidelines for CDSS functionalities [29], while XML encodes knowledge bases for mobile prenatal care CDSS [34].
AI Methodologies and Applications in Automated Risk Assessment
Algorithms: Diverse Approaches to Risk Prediction
Knowledge-independent CDSS often employ computational algorithms for decision boundary learning. Supervised algorithms (classification, prediction) require human-annotated data, while unsupervised algorithms (clustering) identify decision boundaries without explicit labels. Regression-based algorithms are frequently used as benchmarks for clinical prediction, diagnostic support, and treatment recommendations. While some studies use parametric linear statistical models as benchmarks [35], supervised machine learning algorithms like support vector machines, random forests, and gradient boosting (e.g., XGBoost) are increasingly favored for their superior performance. Simpler neural networks (multilayer perceptron, artificial neural networks) are also used, especially when feature spaces are less complex [17, 18, 30]. Ontology embedding is being explored to integrate domain-specific medical knowledge and clinical guidelines into machine learning models [36]. Deep learning algorithms (convolutional neural networks, recurrent neural networks) are also emerging, demonstrating outperformance in certain applications [38].
Knowledge-based CDSS rely on rules (if-then, fuzzy logic) or semantic relations defined by ontologies. Natural Language Processing (NLP) techniques are used with ontology-based knowledge bases for medical image annotation [26, 27]. Rule-based algorithms also offer clinical interpretability [16]. Ontologies are a common choice for knowledge base construction (n=2, 7%) [26, 27].
Performance Evaluation: Ensuring Tool Reliability
The majority of studies (n=28, 93%) have incorporated internal validation, using methods like hold-out, n-fold cross-validation, and bootstrap or cross-validation. Evaluation metrics include precision, recall, F-measure, AUC [48], probabilistic statistics-based metrics (mean squared error, Matthew’s correlation coefficient), descriptive statistics (accuracy), and customized accuracy measures. A few studies reported confidence intervals [33, 35], enhancing result interpretability. External validation was present in 5 studies (16%) [14, 24, 32, 35, 37], typically testing CDSS on independent datasets from different clinical sites.
Addressing Potential Biases in Automated Risk Assessment
Biases can arise in data sampling, processing (handling missing data, normalization), model training, validation, and algorithm design, potentially skewing CDSS performance and clinical decisions. Untrained data and biased training/validation datasets can significantly influence AI-augmented systems, including CDSS. However, existing studies often lack comprehensive discussions on bias mitigation during CDSS design and development. Recognizing and addressing biases is crucial for ensuring equitable and reliable automated risk assessment in pregnancy care.
CDSS Implementation: Bridging Research to Practice
Clinical implementation is the crucial final step for translating research findings into routine practice, yet only a few studies have discussed conceptual implementation ideas or pilot study designs (n=3, 10%) [18, 23, 25]. Implementation strategies included web-based data entry and graphical result presentation [18, 25], and Android-based interfaces [23]. Comprehensive implementation studies, including usability testing, were notably absent in the reviewed literature.
Discussion
Key Findings and Implications for Automated Risk Assessment in Pregnancy Care
The past decades have witnessed a surge in AI applications within clinical medicine, yet AI-augmented CDSS, particularly automated risk assessment tools, in obstetrics and gynecology have not been systematically reviewed until now. This review assessed studies across healthcare applications, CDSS functionality, AI methodology, and clinical implementation to provide a state-of-the-art overview, highlighting advantages, limitations, and future directions. We identified 30 relevant studies published between 1994 and 2022, showing an increasing trend, especially after 2021. Data sources primarily included EHRs, registries, and mobile devices. CDSS functions in pregnancy care encompass diagnostic support (imaging), clinical prediction, treatment recommendations, and knowledge bases. Notably, traditional CDSS functions like patient safety alerts, clinical management prompts, and administrative support were less prevalent in the reviewed studies [49-51]. CDSS architectures were both knowledge-based (using ontologies and NLP) and knowledge-independent (relying on machine learning). Machine learning, ontologies, and NLP are confirmed as increasingly vital components of modern pregnancy care CDSS.
Clinical Implications and Real-World Potential of Automated Risk Assessment
Several factors influence the potential and challenges of adopting AI-augmented CDSS, specifically automated risk assessment tools, in pregnancy care.
Firstly, model performance within individual pregnancy episodes is crucial. Existing CDSS support prenatal, obstetric, and postpartum care, with prenatal applications largely focused on predicting risks like miscarriage [36], ectopic pregnancy [26, 30], gestational diabetes [33, 40], preterm birth [14, 16, 17], and severe maternal morbidity [41]. While predictive performance in these studies is generally strong, the clinical utility hinges on how early a CDSS can reliably detect risk and inform decisions – a critical factor often under-addressed. For obstetrics, CDSS are being developed to assist with delivery mode selection [28, 42], preterm infant extubation [18], and intrapartum diagnostics [22], showing promising potential for clinical integration. In postpartum care, CDSS for postpartum hemorrhage [52] and depression risk detection [23, 31] have demonstrated good performance. However, real-world implementation may face data collection challenges due to limited postpartum patient encounters and mobile app engagement.
Secondly, model interoperability is paramount. Beyond performance, interpretability is key for clinicians to understand model outputs and contributing factors. Knowledge-based CDSS offer inherent interpretability due to traceable knowledge and semantic reasoning. Our review found four studies utilizing biomedical ontologies and semantic web technologies for knowledge bases related to pregnancy risk, antenatal guidelines, ultrasound imaging, and ectopic pregnancy [26, 27, 34, 36]. Predictive CDSS models fall into two categories: parametric (regression models [16, 18, 23, 31-33, 37, 38, 40, 52], decision trees [15, 19, 28, 31, 33, 41], shallow neural networks [17, 18, 23-25, 28, 30, 42], expert systems [14, 15]) and non-parametric/deep learning models [20, 21, 28, 30, 38, 42, 43]. Parametric models offer greater transparency in decision logic, while non-parametric models excel with large, complex datasets but have limited interpretability.
Data availability and quality are significant hurdles for developing pregnancy care CDSS, including automated risk assessment tools. Prenatal data is often fragmented across hospitals, outpatient groups, and labs with varying technologies and data entry protocols. Inconsistent prenatal care initiation and frequency further complicate data collection, leading to potential biases in CDSS training and real-world application, as individuals with late or infrequent care often have poorer outcomes [53].
Strengths, Limitations, and Future Directions for Automated Risk Assessment Tools
Overview of Strengths and Areas for Improvement
This review highlights several strengths in current CDSS design for pregnancy care. However, given the nascent stage of AI-augmented CDSS in this field, we also identified limitations and suggest future directions.
Enhancing Internal Validation Robustness
Existing studies exhibit several strengths in study design: (1) reliance on real-world EHR, registry, and mobile device data, with some studies using multi-center data [14, 17, 23, 37, 39, 43]; (2) generally sufficient sample sizes, although some used smaller samples (n=100-300); (3) comparison of multiple AI algorithms with benchmarks in most studies (n=19, 73%); (4) common use of cross-validation or hold-out methods for internal validation; and (5) use of appropriate performance metrics like F-score, AUC, and probabilistic statistics-based metrics. However, some studies relied solely on accuracy, which is less comprehensive for performance validation. Overall, reported model performance is promising.
Improving Clinical Plausibility and Relevance
(1) Most studies explicitly stated clinical use cases for their CDSS, recognizing early detection of abnormalities and at-risk pregnancies as core clinical benefits. However, data scarcity and challenges in integrating longitudinal, multi-specialty records limit early diagnosis and prediction capabilities. (2) Studies also addressed unique diagnostic and therapeutic challenges in pregnancy care, such as CTG interpretation and delivery mode selection. However, the inter-rater variability in medical interpretations like CTG necessitates repeated evaluations for CDSS design. Furthermore, the long-term impacts of cesarean delivery on both fetal and subsequent maternal outcomes [47] are often overlooked in CDSS design, limiting their clinical value in delivery mode selection. Future research should explore CDSS applications in emergency pregnancy care, a currently under-investigated area.
Mitigating Potential Biases in Automated Risk Assessment
CDSS and automated risk assessment tools can inadvertently introduce biases. (1) Data sampling bias is a significant concern, as with AI algorithms in general. (2) Racial and ethnic disparities in maternal morbidity and mortality [54] necessitate careful consideration of social determinants of health (SDOH). Training CDSS on data from specific socioeconomic groups can introduce biases. The American College of Obstetricians and Gynecologists recommends incorporating SDOH screenings to mitigate bias and improve equitable clinical decision-making [55]. Future CDSS design and implementation must actively address and mitigate these biases, focusing on targeted populations and SDOH.
Emphasizing External Validation and Implementation for Real-World Impact
External validation and implementation remain rare in reviewed CDSS studies, despite their critical importance. Without external validation, the generalizability and clinical usability of these tools are untested. Challenges include CDSS model interoperability and integration with clinical information systems. Successful CDSS implementation requires organizational commitment, workflow adaptation, usability testing, and staff training [56]. The generalizability of machine learning models is a key challenge for wider CDSS adoption. Future research must prioritize external validation and address implementation barriers to realize the full potential of automated risk assessment tools.
Limitations of This Review
This review has limitations. Our search strategy, primarily keyword and MeSH term based, may not have captured all relevant CDSS studies, particularly those not explicitly using CDSS terminology. The loosely defined nature of CDSS itself poses a limitation to our eligibility criteria. Despite these limitations, this review provides timely and valuable insights to guide evidence-based practice and future research directions in pregnancy care CDSS. Our study adheres to PRISMA guidelines and involved dual independent review for study selection and evaluation, enhancing its rigor.
Conclusion
This review provides a comprehensive overview of state-of-the-art AI-augmented CDSS methods and applications, including automated risk assessment tools, in pregnancy care. It highlights the increasing prevalence of machine learning-based predictive models and computer-aided diagnostics and therapeutics, demonstrating promising internal validity. Recent advancements include: (1) CDSS designed for early diagnosis of prenatal abnormalities and risk stratification for timely intervention; (2) CDSS to aid in complex medical image interpretation and decision-making; and (3) the development of knowledge bases for specific pregnancy care domains, such as image annotation, adverse events, and clinical guidelines. Future research should focus on mitigating biases in AI and CDSS, conducting rigorous external validation and implementation studies, and further enhancing the clinical plausibility and utility of these innovative tools to revolutionize pregnancy care.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
None declared.
Multimedia Appendix 1: PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist.
PDF File (Adobe PDF File), 623 KB
Multimedia Appendix 2: Search strings.
References
[References]
Abbreviations
| AI: artificial intelligence |
|---|
| CDSS: clinical decision support systems |
| CTG: cardiotocography |
| EHR: electronic health record |
| MeSH: Medical Subject Headings |
| NLP: natural language processing |
| PMC: PubMed Central |
| PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| SDOH: social determinants of health |
Edited by A Mavragani; submitted 20.11.23; peer-reviewed by S Kommireddy, N Fareed, G Carot-Sans, M Galani; comments to author 06.04.24; revised version received 06.05.24; accepted 24.07.24; published 16.09.24.
Copyright ©Xinnian Lin, Chen Liang, Jihong Liu, Tianchu Lyu, Nadia Ghumman, Berry Campbell. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 16.09.2024.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work, first published in the Journal of Medical Internet Research (ISSN 1438-8871), is properly cited. The complete bibliographic information, a link to the original publication on https://www.jmir.org/, as well as this copyright and license information must be included.


